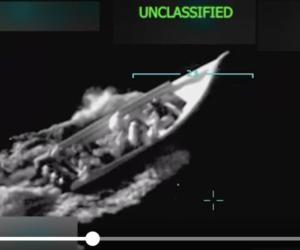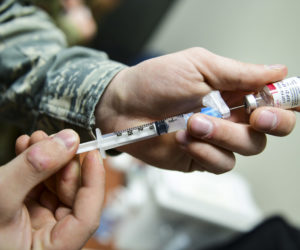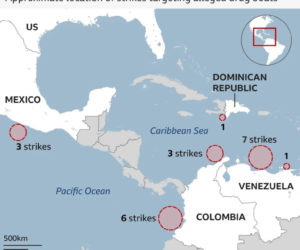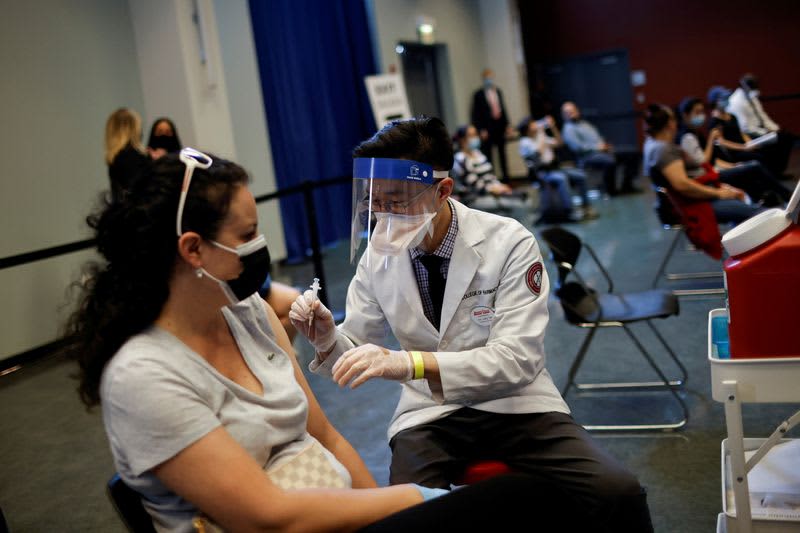
By Reuters staff (Reuters) – U.S. federal health agencies on Tuesday recommended pausing the use of Johnson & Johnson’s COVID-19 vaccine after six recipients developed a rare disorder involving blood clots, in a fresh setback to global efforts to tackle the pandemic. The move comes a week after European regulators said they had found a possible link between AstraZeneca’s COVID-19 vaccine and a rare blood clotting problem that had led to a small number of deaths. Johnson & Johnson’s (J&J) single dose vaccine – most COVID-19 shots are delivered over two doses – and AstraZeneca’s low-cost vaccine…